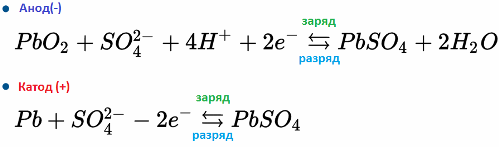Categories: How does it work, Auto electrician
Number of views: 16526
Comments on the article: 0
The device and the principle of battery operation
The electric battery is called reusable chemical current source. The chemical processes inside the battery, in contrast to those in disposable galvanic cells, such as alkaline or salt batteries, are reversible. The cycles of charge-discharge, accumulation and return of electrical energy can be repeated many times.
So, the principle of operation of the battery allows you to cyclically use it for autonomous power supply of a variety of devices, portable devices, vehicles, medical equipment, etc. in completely different areas.

Speaking the word "battery", they mean either the battery itself or the battery cell. Several series or parallel connected to each other battery cells form a battery, as well as several connected batteries.
The first battery, that is, a reusable galvanic cell, appeared, according to official figures, in 1803. It was created by the German physicist and chemist Johann Wilhelm Ritter. A friend of Oersted, Ritter, not being a scientist, studied the chemical effect of light, conducted experiments with electrolysis, by the way, he belongs to the discovery of the ultraviolet part of the electromagnetic spectrum.
While experimenting with a voltaic column, Ritter took fifty circles of copper, pieces of wet cloth, and made up a column of fifty such circles and wet cloth between them. Passing the current from the voltaic column through the structure, Ritter discovered that his pole was charged and itself became a source of electricity. This was the first battery.
The reversibility of the chemical reaction in the electrolyte and on the electrodes of the battery allows you to restore the efficiency of the battery - charge it after discharge. Current during the charge is passed through the battery in the opposite direction to the discharge.
For example, a lead-acid battery operates due to the electrochemical reactions of lead and lead dioxide in sulfuric acid. The formulas below reflect reversible reactions occurring on the anode and on the cathode: from left to right - reaction during discharge, from right to left - charge.
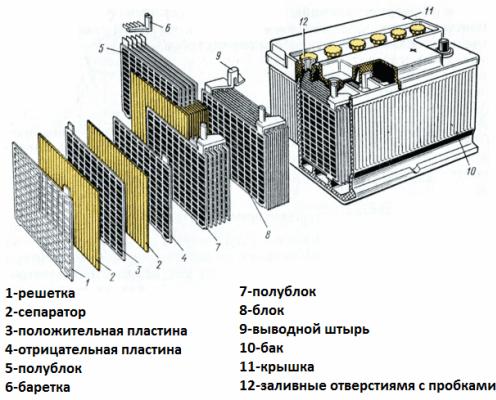
Consider now the battery device as an example of a car starter battery. Its voltage is 12 volts. The battery consists of six cells connected in series, separated by partitions.
Serial connection in this case means that the negative terminal of one cell is connected to the positive terminal of the next cell.
Each element includes a pair of lattice electrodes of a lead-antimony alloy immersed in an electrolyte, which is a 38% aqueous solution of sulfuric acid. The porous separator isolates the electrodes from each other, preventing short circuits between them, but freely passes electrolyte through itself. That is, the liquid fills both the cells of the lead plates and the pores of the separators.
The plates of the same name are interconnected by lead jumpers, as well as the plate packages separated by partitions, which make up the individual elements, and the battery terminals are also made of lead.
The conclusions of the car battery are always slightly different in size from each other - the positive terminal is larger in diameter than the negative one so as not to make a mistake when connecting.
The battery case is made of a dielectric material resistant to aggressive environments, temperature extremes and vibration. Today, starter battery cases are made of polypropylene.
The case is a hermetically sealed container with a lid, equipped with flanges for durable mounting.In the housings of old batteries, plugs were always provided for each of the galvanic cells making up the battery, so that distilled water could be added if necessary. Modern maintenance-free battery plugs on the cases do not have.
Other articles about batteries and their use:
How are batteries for solar power plants
See also at i.electricianexp.com
: