Categories: Featured Articles » Novice electricians
Number of views: 36288
Comments on the article: 1
Chemical current sources: main characteristics
 For more than two centuries, mankind has been using the energy of chemical reactions between various substances to produce direct current.
For more than two centuries, mankind has been using the energy of chemical reactions between various substances to produce direct current.
Principle of operation
The redox reaction occurring between substances with the properties of an oxidizing agent and a reducing agent is accompanied by the release of electrons, the movement of which forms an electric current. However, in order to use its energy, it is necessary to create conditions for the passage of electrons through an external circuit, otherwise it is released into the external environment by simply mixing the oxidizing agent and reducing agent with heat.
Therefore, all chemical current sources have two electrodes:
-
the anode on which oxidation occurs;
-
cathode, carrying out the restoration of the substance.
Electrodes at a distance are placed in a vessel with an electrolyte - a substance that conducts electric current due to the processes of dissociation of the medium into ions.
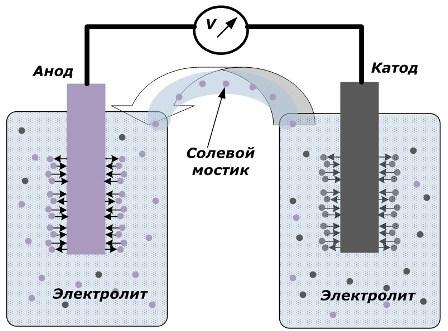
The principle of converting chemical energy into electrical energy
The figure shows that the electrodes are placed in separate vessels connected by a salt bridge through which the movement of ions along the internal circuit is created. When the external and internal circuits are open, two processes occur on the electrodes: the transition of ions from the metal of the electrode to the electrolyte and the transition of ions from the electrolyte to the crystal lattice of the electrodes.
The flow rates of these processes are the same and the voltage potentials of opposite signs are accumulated on each electrode. If they are connected through a salt bridge and a load is applied, an electrical circuit will occur. An internal current is generated by the movement of ions between the electrodes through the electrolyte and the salt bridge. The movement of electrons along the external circuit in the direction from the anode to the cathode.
Almost all redox reactions are accompanied by electricity generation. But its value depends on many factors, including the volumes and masses of the chemicals used, the materials used to make the electrodes, such as electrolyte, ion concentration, design.
The most widely used in modern chemical current sources are:
-
for the material of the anode (reducing agent) - zinc (Zn), lead (Pb), cadmium (Cd) and some other metals;
-
for the cathode material (oxidizer) - lead oxide PbO2, manganese oxide MnO2, nickel hydroxide NiOOH and others;
-
electrolytes based on solutions of acids, alkalis or salts.
Classification Methods
One part of chemical power sources can be reused, while the other cannot. This principle is taken as the basis for their classification.
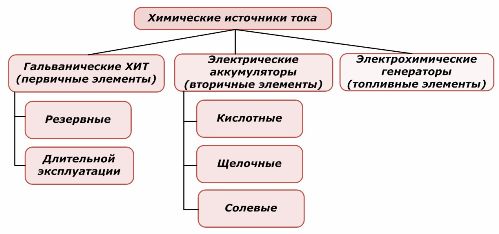
Classification of chemical elements
Electromotive force galvanic cells, depending on the design, reaches 1.2 ÷ 1.5 volts. To obtain large values, they are combined into batteries, connecting in series. When the batteries are connected in parallel, the current and power increase.
It is generally accepted that primary chemical current sources do not support recharging, although more precisely this position can be formulated differently: its implementation is not economically feasible.
Redundant primary chemical current sources are stored in a state where the electrolyte is isolated from the electrodes. This eliminates the occurrence of the redox reaction and ensures readiness for commissioning. They are not reused. The shelf life of chemical backup power sources is limited to 10–15 years.
Batteries are successfully recharged by the application of external electrical energy. Due to this feature, they are called secondary current sources. They are able to withstand hundreds and thousands of charge-discharge cycles.The EMF of the battery can be in the range of 1.0 ÷ 1.5 volts. They are also combined into batteries.
Electrochemical generators work on the principle of galvanic cells, but to carry out the electrochemical reaction, substances come from outside, and all released products are removed from the electrolyte. This allows you to organize a continuous process.
Key performance characteristics of chemical power sources
1. The voltage across open terminals
Depending on the design, a single source can create only a certain potential difference. For use in electrical devices, they are combined into batteries.
2. Specific capacity
For a certain time (in hours) one chemical current source can generate a limited amount of current (in amperes), which are attributed to a unit of weight or volume.
3. Power density
It characterizes the ability of a unit of weight or volume of a chemical current source to generate power generated by the product of voltage by current strength.
4. Duration of operation
This parameter is also called the expiration date.
5. The value of self-discharge currents
These side processes of electrochemical reactions lead to the consumption of the active mass of the elements, cause corrosion, and reduce the specific capacity.
6. Product price
Depends on the design, materials used and a number of other factors.
The best chemical current sources are those with high values of the first four parameters, and self-discharge and cost are low.
Battery Charge Principles
Among secondary chemical current sources, they are gaining great popularity. lithium ion models, which have become widely used to power electronic devices. They use LiMO2 (M Co, Ni, Mn) as the material of the positive electrode, and graphite as the negative electrode.
When charged, lithium ions from the applied external energy are released from the cathode metal, pass through the electrolyte and penetrate into the space between the graphite layers, accumulating there.
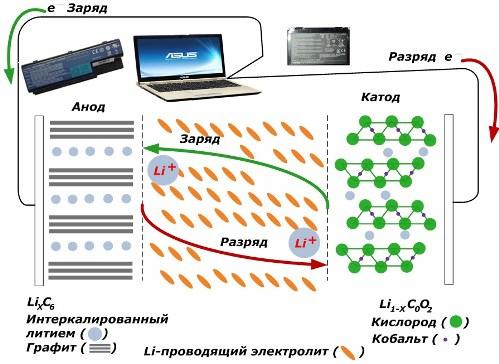
When the energy charger is absent, and the load is connected to the electrodes, then lithium ions in the electrolyte move in the opposite direction.
If the charge and discharge are not carried out, then the energy in the battery is not consumed, but stored. But its quantity is limited by the properties of the materials used. For example, in lithium-ion batteries, the specific electric capacity is 130 ÷ 150 mAh / g. It is limited by the properties of the anode material. For graphite, the capacity is about two times higher.
Scientists are now looking for ways to increase battery capacity, are exploring the possibility of using a chemical reaction between lithium and oxygen in the air. To do this, designs are developed with an air, non-expendable cathode, used in separate batteries. This method can increase energy density up to 10 times.
The operation of chemical current sources requires knowledge fundamentals of electrical engineering, electrochemistry, materials science and solid state physics.
See also at bgv.electricianexp.com
:
