Categories: Featured Articles » Interesting Facts
Number of views: 14188
Comments on the article: 1
Why do batteries explode
 Users of smartphones and tablets are of course aware of the explosion hazard of lithium batteries in their gadgets. And striking examples do not have to go far. Recently, for example, Samsung faced a painful problem personally, and was forced to recall the first series of the new Note 7, since the batteries exploded right in the process of charging. One way or another, the problem remains the same from the beginning of the appearance of cell phones; even in 2016, ICAO banned commercial shipments in the cargo compartments of civilian transport lithium batteries.
Users of smartphones and tablets are of course aware of the explosion hazard of lithium batteries in their gadgets. And striking examples do not have to go far. Recently, for example, Samsung faced a painful problem personally, and was forced to recall the first series of the new Note 7, since the batteries exploded right in the process of charging. One way or another, the problem remains the same from the beginning of the appearance of cell phones; even in 2016, ICAO banned commercial shipments in the cargo compartments of civilian transport lithium batteries.
The essence of the problem with lithium batteries
The fact is that in the process of charging a lithium battery in a mobile device, using the microcontroller built into the battery, a rather complicated algorithm for implementing this process is implemented so that the battery temperature does not go beyond the acceptable temperature range. The controller monitors for this purpose many parameters of the battery during its charging.
In addition to the charging process itself, storage of the battery also requires compliance with certain rules, especially regarding temperature: you can neither overheat nor supercool the battery.

The main problem causing batteries to explode is excessive heating of the electrolyte due to exceeding the permissible temperature or due to a short circuit inside the battery cell. The chain reaction is easily initiated inside the overheated cell, because the alkali metal lithium is very easily ignited, as a result of which the battery swells and, in the worst case, explodes.
And even despite the presence of an “attentive” controller, an accidental factory defect (insufficient thickness of the insulator between the cells) can take place and lead to sad consequences.
Of course, shock, breakdowns, punctures, overheating in the sun are dangerous. Even if the battery has fallen and hit slightly, a breakdown of the insulator can occur inside, and in the future this may lead to a sudden trouble, even without obvious overheating.
Explosion Reason for Lithium Batteries
The anode and cathode of the lithium-ion battery are separated by a porous polymer separator. The cathode has an active material on it, for which oxides of transition metals are often used, in which lithium ions are embedded. The anode is usually graphite. An organic solution of lithium salts is used as an electrolyte.
During the first charge at the plant, lithium is built into the anode and a layer of decomposed electrolyte forms on the electrodes, which now serves as protection against unnecessary reactions, while remaining ion-conducting.
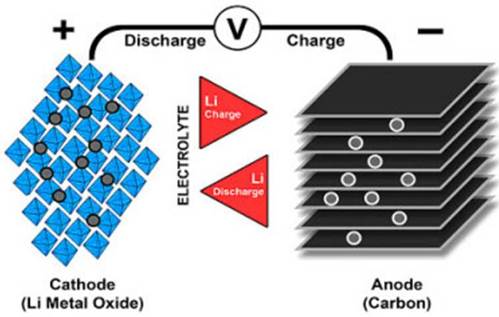
As noted above, an internal short circuit is one of the main causes of battery self-ignition. The cause of the short circuit itself may be physical damage or factory defects, such as uneven cutting of the electrodes or the ingress of metal particles between the cathode and anode, which violate the integrity of the separator layer.
Another reason for the closure is the growth of lithium metal chains through the separator (if the lithium ions at the factory did not have enough time to fully integrate into the anode crystal due to excessively fast charging or from overcooling, or if the capacity of the cathode active material is larger than the capacity of the anode, which leads to deposits on the anode, which then slowly but inexorably grow).
So, if a short circuit occurred, then the battery temperature begins to rise, and when it reaches 70-90 ° C, the decomposition of the protective ion-conducting layer of the anode begins. The lithium anode reacts with an electrolyte, while combustible hydrocarbons such as ethylene, methane, ethane, etc. are released.But it’s too early before the fire, because there is not enough oxygen.
Meanwhile, the exothermic reaction is on and the temperature rises, the pressure inside the battery case rises. At 180-200 ° C, the disproportionation reaction begins at the cathode, where oxygen is released. Ignition occurs, the temperature rises sharply, and the electrolyte decomposes thermally, the temperature is already 200-300 ° C.
Finally, it is the turn of graphite, and when the temperature reaches 660 ° C, the aluminum of the current collector begins to melt. The maximum temperature in this whole process usually does not have time to exceed 900 ° C, since everything quickly ends with the complete decomposition of the internal components of the battery.

Already there is success in finding a solution to the problem
To solve the problem, smartphone manufacturers can tighten regulation, make additional fuses in devices and in batteries, complicate controllers, but this will increase the cost of batteries and all products that come with a battery. Companies compete with each other, and simply economically can not do it.
Meanwhile, physicists from Stanford are fighting for the safety of lithium batteries, who back in the summer of 2015 developed a special protective mechanism that is built into the battery already at the production stage.
In fact, we are talking about a new type of lithium batteries, which automatically turn off when their inside reaches a potentially dangerous temperature (which prevents the process leading to subsequent fire), and after a while, after cooling, they turn on again automatically.
The authors of the development claim that this is the first lithium battery that can repeatedly turn off and restore without losing its properties and performance.
The development was carried out for several years by a team of several people (including Zhenan Bao), as a result, a battery was devoid of two main drawbacks - a sharp decrease in battery capacity after several recharging cycles and, more importantly, a tendency to fire and explosion due to overheating ( chain reaction stops automatically).
The decision came to scientists from a completely different field of physics. They made thermometers using nickel nanoparticles embedded in a thin sheet of graphene and plastic. These were unusual thermometers. At rest, the nickel particles were in contact with each other, that is, a good current conductor was obtained. But when the sheet was heated, the plastic began to expand slightly, which led to a weakening of the contact between the conductive nickel particles, and the resistance of the entire conductor increased.
This property was used by researchers from Stanford for instant automatic protection of lithium batteries and for full automatic restoration of contact after cooling. They glued a sheet of such plastic to one of the electrodes of the battery so that it would lose conductivity with increasing temperature. And when the temperature reaches 70 ° C

But despite the solution, the manufacturers of mobile devices still do not dare to drastically change the production technology of their batteries that has been developed over the years. Therefore, gadget users will have to come to terms with the potential danger of lithium batteries for some time, and try not to drop or overheat their mobile devices, and especially batteries. Perhaps in the near future the problem will be completely resolved.
See also: Proper use of lithium-ion batteries
See also at bgv.electricianexp.com
:
