Categories: Featured Articles » Novice electricians
Number of views: 22508
Comments on the article: 3
Battery internal resistance
If we take a brand-new lithium-ion battery, let us say size 18650, with a nominal capacity of 2500mAh, bring its voltage to exactly 3.7 volts, and then connect it to an active load in the form of a 10-watt resistor with a value of R = 1 Ohm, then what is the constant current we expect to measure through this resistor?

What will happen there at the very first moment of time, until the battery almost starts to discharge? In accordance with Ohm's law, it would seem that there should be 3.7A, since i = U / R = 3.7 / 1 = 3.7 [A]. In fact, the current will be slightly less, namely, in the region of I = 3.6A. Why is this going to happen?
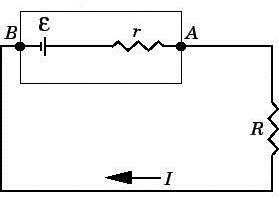
The reason is that not only the resistor, but the battery itself has a certain internal resistance, because the chemical processes inside it cannot occur instantly. If you imagine a battery in the form of a real two-terminal, then 3.7V - this will be its EMF, in addition to which there will also be an internal resistance r equal to, for our example, approximately 0.028 Ohm.
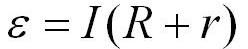
Indeed, if you measure the voltage on a resistor connected to the battery with a value of R = 1 Ohm, then it turns out to be approximately 3.6 V, and 0.1 V will therefore fall on the internal resistance r of the battery. So, if the resistor has a resistance of 1 ohm, the voltage measured on it was 3.6 V, therefore the current through the resistor is I = 3.6 A. Then, if u = 0.1 V fell on the battery, and the circuit we have is closed, series, it means that the current through the battery is I = 3.6 A, therefore, according to Ohm's law, its internal resistance will be equal to r = u / I = 0.1 / 3.6 = 0.0277 ohms.
What determines the internal resistance of the battery
In reality, the internal resistance of different types of batteries is not always constant. It is dynamic, and depends on several parameters: on the load current, on the battery capacity, on the degree of charge of the battery, as well as on the temperature of the electrolyte inside the battery.
The higher the load current, the less, as a rule, the internal resistance of the battery, since the processes of charge transfer inside the electrolyte are more intense in this case, more ions are involved in the process, ions move more actively in the electrolyte from the electrode to the electrode. If the load is relatively small, then the intensity of the chemical processes at the electrodes and in the battery electrolyte will also be less, and therefore the internal resistance will seem large.
For batteries with a larger capacity, the area of the electrodes is larger, which means that the area of interaction of the electrodes with the electrolyte is more extensive. Therefore, more ions are involved in the process of charge transfer, more ions create a current. A similar principle is demonstrated. with parallel connection of capacitors - the larger the capacity, the more charge can be used in the vicinity of a given voltage. So, the higher the battery capacity - the lower its internal resistance.
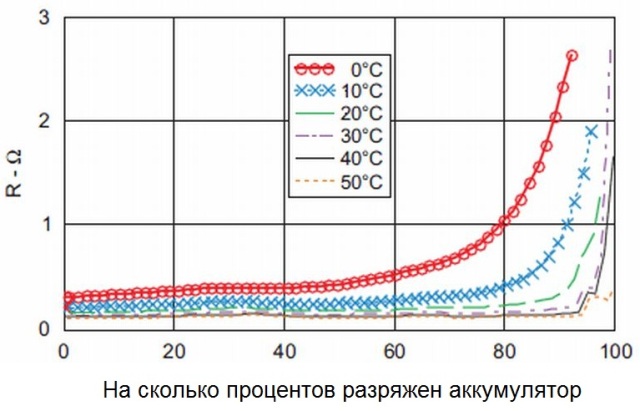
Now let's talk about temperature. Each battery has its own safe operating temperature range, within which the following is true. The higher the temperature of the battery, the faster the diffusion of ions inside the electrolyte occurs, therefore, at a higher operating temperature, the internal resistance of the battery will be lower.
The first lithium batteries, which did not have protection against overheating, even exploded because of this, since oxygen formed too actively was formed due to the rapid decay of the anode (as a result of a quick reaction on it). One way or another, batteries are characterized by an almost linear dependence of internal resistance on temperature in the range of acceptable operating temperatures.
With the discharge of the battery, its active capacity decreases, since the amount of active substance of the plates, still able to participate in the creation of current, becomes less and less. Therefore, the current is becoming less and less, respectively, the internal resistance is growing. The more charged the battery, the less its internal resistance. So, as the battery discharges, its internal resistance becomes greater.
See also at bgv.electricianexp.com
:
