Categories: Featured Articles » Novice electricians
Number of views: 70526
Comments on the article: 0
Galvanic cells - device, principle of operation, types and main characteristics
Prerequisites for the appearance of galvanic cells. A bit of history. In 1786, the Italian professor of medicine, physiologist Luigi Aloisio Galvani discovered an interesting phenomenon: the muscles of the hind legs of a freshly opened corpse of a frog suspended on copper hooks contracted when the scientist touched them with a steel scalpel. Galvani immediately concluded that this was a manifestation of "animal electricity."
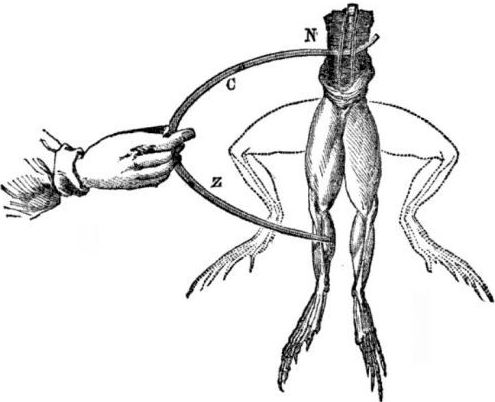
After the death of Galvani, his contemporary Alessandro Volta, as a chemist and physicist, will describe and publicly demonstrate a more realistic mechanism for the occurrence of electric current when different metals come into contact.
Volta, after a series of experiments, will come to the unequivocal conclusion that the current appears in the circuit due to the presence in it of two conductors of different metals placed in a liquid, and this is not at all “animal electricity”, as Galvani thought. The twitching of the frog's legs was a consequence of the action of the current arising from the contact of various metals (copper hooks and a steel scalpel).
Volta will show the same phenomena that Galvani showed on a dead frog, but on a completely inanimate home-made electrometer, and in 1800 will give an accurate explanation of the current: “the second-class conductor (liquid) is in the middle and is in contact with two first-class conductors of two different metals ... As a result of this, an electric current of one direction or another arises. ”

In one of the first experiments, Volta lowered two plates — zinc and copper — into a jar of acid and connected them with wire. After that, the zinc plate began to dissolve, and gas bubbles formed on the copper steel. Volta suggested and proved that electric current flows through the wire.
Thus was invented the "Volta element" - the first galvanic cell. For convenience, Volta gave it the shape of a vertical cylinder (pillar), consisting of interconnected rings of zinc, copper and cloth impregnated with acid. A volt pole half a meter high created a voltage sensitive to humans.
Since the beginning of the research was laid by Luigi Galvani, the name chemical current source preserved the memory of him in his name.
Galvanic cell Is a chemical source of electric current, based on the interaction of two metals and / or their oxides in an electrolyte, leading to the appearance of an electric current in a closed circuit. Thus, in galvanic cells, chemical energy is converted into electrical energy.
Cells Today

Cells today are called batteries. Three types of batteries are widespread: saline (dry), alkaline (they are also called alkaline, "alkaline" in translation from English - "alkaline") and lithium. The principle of their work is the same as described by Volta in 1800: two metals interact through electrolyte, and an electric current arises in an external closed circuit.
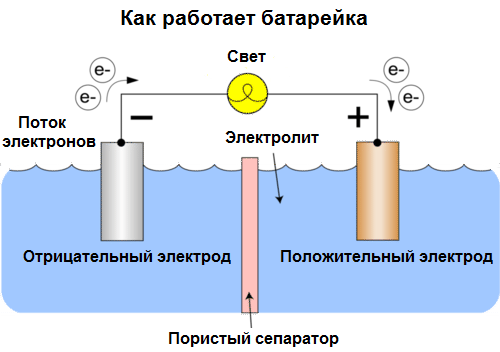
Battery voltage depends on the metals used, and on the number of cells in the "battery". Batteries, unlike batteries, are not capable of restoring their properties, since they directly convert chemical energy, that is, the energy of the reagents (reductant and oxidizer) that make up the battery, into electrical energy.
The reagents included in the battery are consumed during its operation, the current gradually decreases, so the action of the source ends after the reagents completely react.
Alkaline and salt elements (batteries) are widely used to power a variety of electronic devices, radio equipment, toys, and lithium can most often be found in portable medical devices such as blood glucose meters or in digital technology such as cameras.

Salt batteries
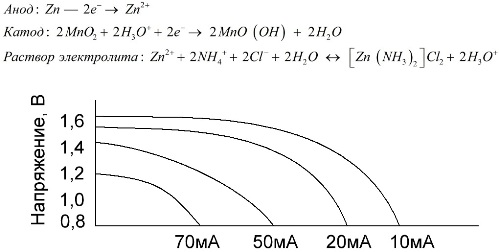
Manganese-zinc cells, which are called salt batteries, are “dry” galvanic cells, inside of which there is no liquid electrolyte solution.
A zinc electrode (+) is a cup-shaped cathode, and a powdery mixture of manganese dioxide and graphite serves as the anode. Current flows through a graphite rod. As an electrolyte, a paste is used from a solution of ammonium chloride with the addition of starch or flour to thicken, so that nothing flows.
Typically, battery manufacturers do not indicate the exact composition of the salt elements, however, salt batteries are the cheapest, they are usually used in devices where power consumption is extremely low: in hours, in remote controls, in electronic thermometers, etc.

The concept of "nominal capacity" is rarely used to characterize manganese-zinc batteries, since their capacity is highly dependent on operating conditions and conditions. The main disadvantages of these elements are a significant rate of voltage reduction throughout the discharge and a significant decrease in the output capacitance with increasing discharge current. The final discharge voltage is set depending on the load in the range of 0.7-1.0 V.
It is important not only the magnitude of the discharge current, but also the time schedule of the load. With intermittent discharges of large and medium currents, the performance of the batteries increases markedly compared to continuous operation. However, with small discharge currents and months of interruptions in operation, their capacitance may decrease as a result of self-discharge.
The graph above shows the discharge curves for an average salt battery for 4, 10, 20, and 40 hours for comparison with an alkaline one, which will be discussed later.
Alkaline (alkaline) batteries
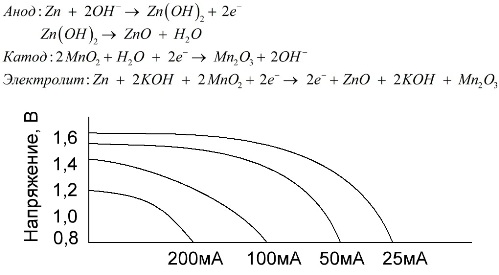
An alkaline battery is a manganese-zinc galvanic battery, in which manganese dioxide is used as a cathode, powdered zinc is used as an anode, and an alkali solution is used as an electrolyte, usually in the form of a potassium hydroxide paste.
These batteries have a number of advantages (in particular, a significantly larger capacity, better performance at low temperatures and high load currents).

Alkaline batteries, in comparison with salt ones, can provide more current for a long time. A greater current becomes possible because zinc is used here not in the form of a glass, but in the form of a powder having a larger contact area with the electrolyte. Potassium hydroxide in the form of a paste is used as an electrolyte.
It is thanks to the ability of this type of galvanic cells to deliver a significant current (up to 1 A) for a long time, alkaline batteries are most common at present.
In electric toys, in portable medical equipment, in electronic devices, in cameras, alkaline batteries are used everywhere. They serve 1.5 times longer than saline if the discharge is low current. The graph shows the discharge curves at different currents for comparison with a salt battery (the graph was given above) for 4, 10, 20 and 40 hours.
Lithium batteries
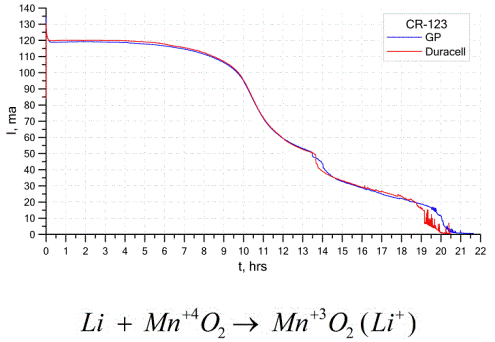
Another fairly common type of galvanic cells is lithium batteries - single non-rechargeable galvanic cells in which lithium or its compounds are used as an anode. Due to the use of alkali metal, they have a high potential difference.

The cathode and electrolyte of a lithium cell can be very different, so the term “lithium cell” combines a group of cells with the same anode material.As a cathode, for example, manganese dioxide, carbon monofluoride, pyrite, thionyl chloride, etc. can be used.
Lithium batteries are distinguished from other batteries by their high runtime and high cost. Depending on the selected size and the chemical materials used, a lithium battery can produce voltages from 1.5 V (compatible with alkaline batteries) to 3.7 V.
These batteries have the highest capacity per unit mass and long shelf life. Lithium cells are widely used in modern portable electronic equipment: for powering watches on computer motherboards, for powering portable medical devices, watches, calculators, in photographic equipment, etc.
The graph above shows the discharge curves for two lithium batteries from two popular manufacturers. The initial current was 120 mA (per resistor of the order of 24 Ohms).
See also: Modern rechargeable batteries - advantages and disadvantages
See also at bgv.electricianexp.com
: