Categories: Featured Articles » Interesting Facts
Number of views: 9906
Comments on the article: 0
Inertia of an electron: Tolman – Stuart and Mandelstam – Papaleksi experiments
Experiments in order to find the answer to the question of whether electrons have an inert mass were carried out by scientists in the very beginning of the 20th century. These experiments helped the scientific community of that time to establish itself in accepting the fact that the electric current in metals is formed precisely by negatively charged particles - electrons, rather than positively charged ions, as one might assume.

The first qualitative experiment, which illustrated that the charged particles forming the electric current in metals precisely possess mass, was carried out by scientists (then the Russian Empire) Leonid Isaakovich Mandelstam and Nikolai Dmitrievich Papaleksi, this took place in 1913.
Three years later, in 1916, a more accurate experiment was conducted by American physicists Richard Tolman and Thomas Stewart, who in their work not only showed that the electron has a mass in a metal, but also accurately measured it by an indirect method using a galvanometer.
To understand the principle of these early experiments, imagine a tram on which passengers go to work early in the morning. Here the tram was dispersed as it should, and in front of it a scattered pedestrian runs out right on the way.
The tram driver, wanting to save the poor fellow's life, sharply presses on the brakes - passengers in the passenger compartment are instantly blown away by the whole crowd. And it blows them with the force of inertia, because each passenger has a mass. And those passengers who were closest to the tram cabin will hit the wall painfully.
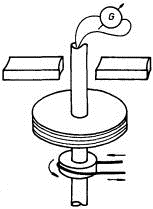
Mandelstam and Papaleksi thought in approximately the same way. They took a coil of wire, equipped with sliding contacts its conclusions isolated from the case, and connected a speaker (earphone) to the sliding contacts. They unwound the coil to the right - abruptly stopped - a click sounded in dynamics.
Twisted to the left - sharply braked - click again in dynamics. Conclusion: at the moment of stopping the coil, a current pulse passes through its wire, which appears due to the fact that the electrons at the time of braking of the coil are discarded to the edge of the wire, like passengers in a tram.
And the force of inertia here plays the role of an external force, which creates what can be measured as EMF. This conclusion, of course, did not allow researchers to recognize the sign of charge carriers and somehow uniquely identify them, however, the experiment by Mandelstam and Papaleksi clearly showed that the current in metals keeps its way through the crystal lattice, which means that it is connected with the free charge carriers.
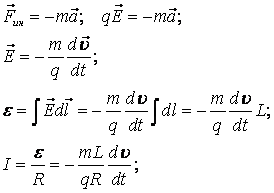
Tolman and Stuart decided to go a little further. They also wound the coil, only the length of the wire was measured exactly equal to 500 meters, and began to unwind it. It was untwisted until a linear velocity of exactly 500 m / s was reached in order to know the ratio between the obtained emf and acceleration.
Already not a speaker, but a more informative device, a galvanometer, was connected to the sliding terminals of the coil. At the end of the experiment, the researchers integrated the extraneous force along the entire length of the coil conductor, and obtained an expression for the EMF created by the extraneous inertia force when the speed changes to zero.

The total charge that ran through the conductor could be calculated according to Ohm's law, taking into account the resistance of the coil wire. So, knowing the speed of the wire before braking, the length of the wire, its resistance, direction of rotation, braking time, magnitude and sign of the emf, you can find the sign and magnitude of the specific charge, which was done by Stuart and Tolman.
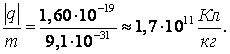
Today it no longer seems strange to anyone that the ratio of electron charge to mass measured by Stuart and Tolman coincided with that obtained almost 20 years ago, in 1897 by J.J. Thomson, the specific charge of the particles that made up the cathode rays. We now probably know that both the cathode rays and the current in metals are formed from the same negatively charged elementary particles - electrons.
See also at bgv.electricianexp.com
:
